Exhibit 99.1

Ola EBV+ PTLD Advocate ATARA BIO® Investor Presentation JP Morgan Healthcare Conference January 10, 2022 Nasdaq: ATRA
Exhibit 99.1

Ola EBV+ PTLD Advocate ATARA BIO® Investor Presentation JP Morgan Healthcare Conference January 10, 2022 Nasdaq: ATRA

Forward-Looking Statements This presentation and the accompanying oral presentation contain forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical facts contained in this presentation, including statements regarding our future results of operations and financial position, business strategy, product candidates, correspondence with regulatory authorities, regulatory submissions, regulatory approvals, the initiation, timing, progress and results of preclinical studies and clinical trials and our research and development programs, ability to sell, manufacture or otherwise commercialize our product candidates, research and development costs, timing and likelihood of success, plans and objectives of management for future operations, any royalty payments, and our ability to obtain and maintain intellectual property protection for our product candidates, are forward-looking statements of Atara Biotherapeutics, Inc. (“Atara” or the “Company”). These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. In some cases you can identify these statements by forward-looking words such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “could,” “would,” “project,” “predict,” “plan,” “expect” or the negative or plural of these words or similar expressions. These forward-looking statements are subject to risks and uncertainties, including those discussed in Atara’s filings with the Securities and Exchange Commission (SEC), including in the “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of the Company’s most recently filed periodic reports on Form 10-K and Form 10-Q and subsequent filings and in the documents incorporated by reference therein. These risks and uncertainties include, without limitation, risks and uncertainties associated with the costly and time-consuming pharmaceutical product development process and the uncertainty of clinical success; the COVID-19 pandemic, which may significantly impact (i) our business, research, clinical development plans and operations, including our operations in South San Francisco and Southern California and at our clinical trial sites, as well as the business or operations of our third-party manufacturer, contract research organizations or other third parties with whom we conduct business, (ii) our ability to access capital, and (iii) the value of our common stock; the sufficiency of our cash resources and need for additional capital, and other factors that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from those anticipated by the forward-looking statements. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. Certain information contained in this presentation and statements made orally during this presentation relate to or are based on studies, publications, surveys and other data obtained from third-party sources and Atara’s own internal estimates and research. While Atara believes these third-party studies, publications, surveys and other data to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, no independent source has evaluated the reasonableness or accuracy of Atara’s internal estimates or research and no reliance should be made on any information or statements made in this presentation relating to or based on such internal estimates and research. The content of this presentation is subject to copyright, which will be asserted by Atara and no part of this presentation may be reproduced, stored in a retrieval system, or transmitted in any form or by any means without prior permission in writing from Atara. ATARA BIO® 2
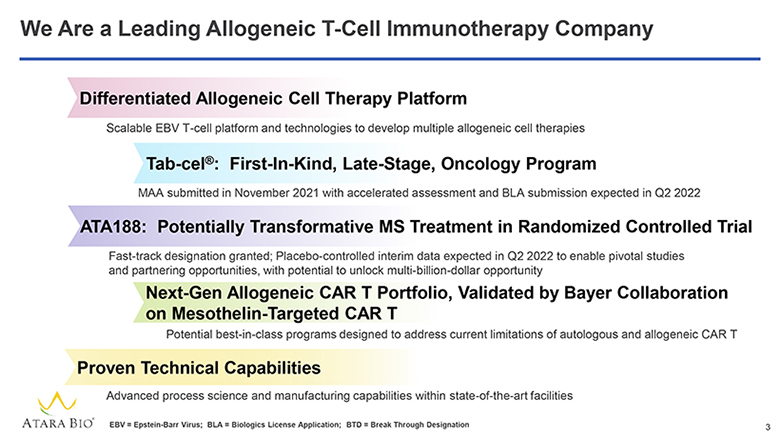
We Are a Leading Allogeneic T-Cell Immunotherapy Company Differentiated Allogeneic Cell Therapy Platform Scalable EBV T-cell platform and technologies to develop multiple allogeneic cell therapies Tab-cel®: First-In-Kind, Late-Stage, Oncology Program MAA submitted in November 2021 with accelerated assessment and BLA submission expected in Q2 2022 ATA188: Potentially Transformative MS Treatment in Randomized Controlled Trial Fast-track designation granted; Placebo-controlled interim data expected in Q2 2022 to enable pivotal studies and partnering opportunities, with potential to unlock multi-billion-dollar opportunity Next-Gen Allogeneic CAR T Portfolio, Validated by Bayer Collaboration on Mesothelin-Targeted CAR T Potential best-in-class programs designed to address current limitations of autologous and allogeneic CAR T Proven Technical Capabilities Advanced process science and manufacturing capabilities within state-of-the-art facilities ATARA BIO® EBV = Epstein-Barr Virus; BLA = Biologics License Application; BTD = Break Through Designation 3
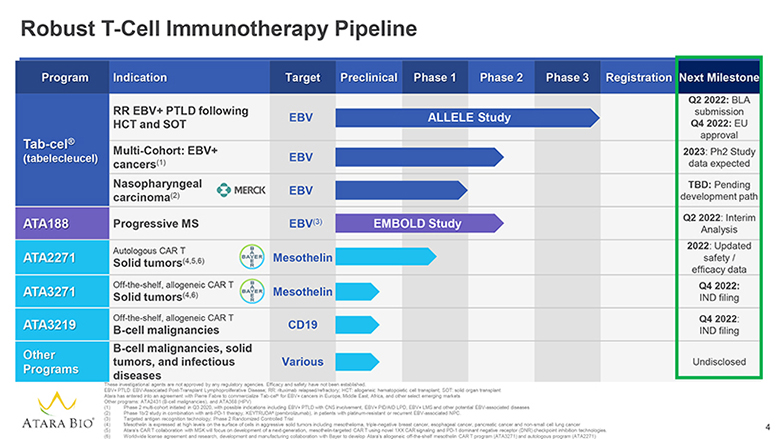
Robust T-Cell Immunotherapy Pipeline Program Indication Target Preclinical Phase 1 Phase 2 Phase 3 Registration Next Milestone Tab-cel® tabelecleucel) RR EBV+ PTLD following HCT and SOT EBV ALLELE Study Q2 2022: BLA submission Q4 2022: EU approval Multi-Cohort: EBV+ cancers(1) EBV 2023: Ph2 Study data expected Nasopharyngeal carcinoma(2) MERCK EBV TBD: Pending development path ATA188 Progressive MS EBV(3) EMBOLD Study Q2 2022: Interim Analysis ATA2271 Autologous CAR T Solid tumors(4,5,6) BAYER Mesothelin 2022: Updated safety / efficacy data ATA3271 Off-the-shelf, allogeneic CAR T Solid tumors(4,6) BAYER Mesothelin Q4 2022: IND filing ATA3219 Off-the-shelf, allogeneic CAR T B-cell malignancies CD19 Q4 2022: IND filing Other Programs B-cell malignancies, solid tumors, and infectious diseases Various Undisclosed ATARA BIO® These investigational agents are not approved by any regulatory agencies. Efficacy and safety have not been established. EBV+ PTLD: EBV-Associated Post-Transplant Lymphoproliferative Disease; RR: rituximab relapsed/refractory; HCT: allogeneic hematopoietic cell transplant; SOT: solid organ transplant Atara has entered into an agreement with Pierre Fabre to commercialize Tab-cel® for EBV+ cancers in Europe, Middle East, Africa, and other select emerging markets Other programs: ATA2431 (B-cell malignancies), and ATA368 (HPV) (1) Phase 2 multi-cohort initiated in Q3 2020, with possible indications including EBV+ PTLD with CNS involvement, EBV+ PID/AID LPD, EBV+ LMS and other potential EBV-associated diseases (2) Phase 1b/2 study in combination with anti-PD-1 therapy, KEYTRUDA® (pembrolizumab), in patients with platinum-resistant or recurrent EBV-associated NPC. (3) Targeted antigen recognition technology; Phase 2 Randomized Controlled Trial (4) Mesothelin is expressed at high levels on the surface of cells in aggressive solid tumors including mesothelioma, triple-negative breast cancer, esophageal cancer, pancreatic cancer and non-small cell lung cancer (5) Atara’s CAR T collaboration with MSK will focus on development of a next-generation, mesothelin-targeted CAR T using novel 1XX CAR signaling and PD-1 dominant negative receptor (DNR) checkpoint inhibition technologies. (6) Worldwide license agreement and research, development and manufacturing collaboration with Bayer to develop Atara’s allogeneic off-the-shelf mesothelin CAR T program (ATA3271) and autologous program (ATA2271) 4
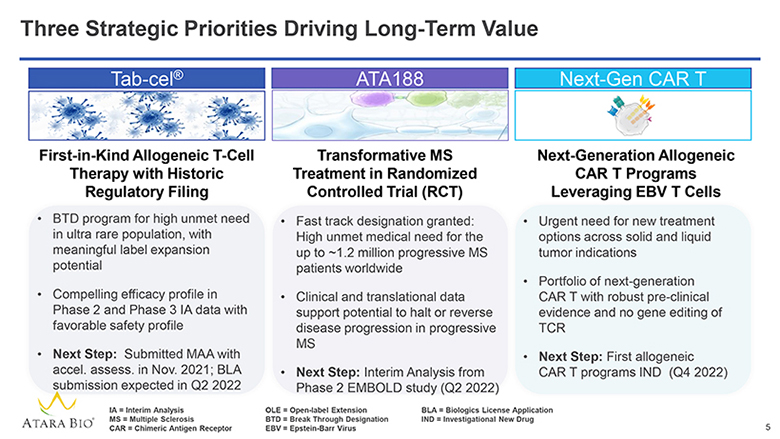
Three Strategic Priorities Driving Long-Term Value Tab-cel® First-in-Kind Allogeneic T-Cell Therapy with Historic Regulatory Filing BTD program for high unmet need in ultra rare population, with meaningful label expansion potential Compelling efficacy profile in Phase 2 and Phase 3 IA data with favorable safety profile Next Step: Submitted MAA with accel. assess. in Nov. 2021; BLA submission expected in Q2 2022 ATA188 Transformative MS Treatment in Randomized Controlled Trial (RCT) Fast track designation granted: High unmet medical need for the up to ~1.2 million progressive MS patients worldwide Clinical and translational data support potential to halt or reverse disease progression in progressive MS Next Step: Interim Analysis from Phase 2 EMBOLD study (Q2 2022) Next-Gen CAR T Next-Generation Allogeneic CAR T Programs Leveraging EBV T Cells Urgent need for new treatment options across solid and liquid tumor indications Portfolio of next-generation CAR T with robust pre-clinical evidence and no gene editing of TCR Next Step: First allogeneic CAR T programs IND (Q4 2022) ATARA BIO® IA = Interim Analysis MS = Multiple Sclerosis CAR = Chimeric Antigen Receptor OLE = Open-label Extension BTD = Break Through Designation EBV = Epstein-Barr Virus BLA = Biologics License Application IND = Investigational New Drug 5
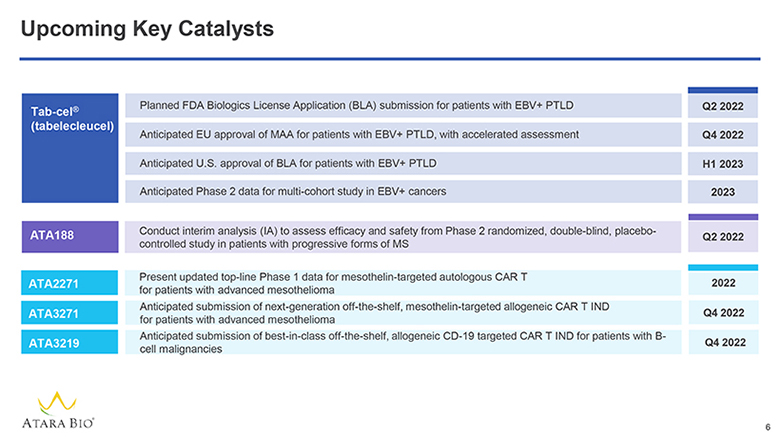
Upcoming Key Catalysts Tab-cel® (tabelecleucel) Planned FDA Biologics License Application (BLA) submission for patients with EBV+ PTLD Q2 2022 Anticipated EU approval of MAA for patients with EBV+ PTLD, with accelerated assessment Q4 2022 Anticipated U.S. approval of BLA for patients with EBV+ PTLD H1 2023 Anticipated Phase 2 data for multi-cohort study in EBV+ cancers 2023 ATA188 Conduct interim analysis (IA) to assess efficacy and safety from Phase 2 randomized, double-blind, placebo-controlled study in patients with progressive forms of MS Q2 2022 ATA2271 Present updated top-line Phase 1 data for mesothelin-targeted autologous CAR T for patients with advanced mesothelioma 2022 ATA3271 Anticipated submission of next-generation off-the-shelf, mesothelin-targeted allogeneic CAR T IND for patients with advanced mesothelioma Q4 2022 ATA3219 Anticipated submission of best-in-class off-the-shelf, allogeneic CD-19 targeted CAR T IND for patients with B-cell malignancies Q4 2022 ATARA BIO® 6
Atara Strategic Priorities to Create Value: Tab-cel® Tab-cel® (tabelecleucel) Investigational T-cell immunotherapy for EBV-associated ultra-rare diseases FDA breakthrough designation and EMA PRIME for EBV+ PTLD MAA submitted in EU with accelerated assessment ATA188 CAR T ATARA BIO® 7
Recent Positive Regulatory Progress for Tab-cel® EUROPEAN MEDICINES AGENCY SCIENCE MEDICINES HEALTH Based on the requests from the FDA following our recent interactions, Atara has provided to the Agency additional analyses of CMC data we have already provided FDA has not requested additional clinical studies or manufacturing lots Atara has made recent progress with FDA through constructive engagement with CBER in Q4 2021 Atara subsequently plans to have further interactions with the FDA through a Type B CMC meeting in Q1 2022 and complete the BLA submission for tab-cel in Q2 2022 Atara continues to adapt investment in U.S. commercial readiness toward anticipated U.S. launch in H1 2023 Following successful interactions with European Medicines Agency (EMA), Atara submitted a MAA for tab-cel® in patients with EBV+ PTLD in November 2021 With the granting of accelerated assessment, anticipate a decision regarding EU tab-cel approval in Q4 2022 ATARA BIO® BLA = Biologics License Application; MAA = Marketing Authorization Application; CMC = Chemistry, Manufacturing and Control 8
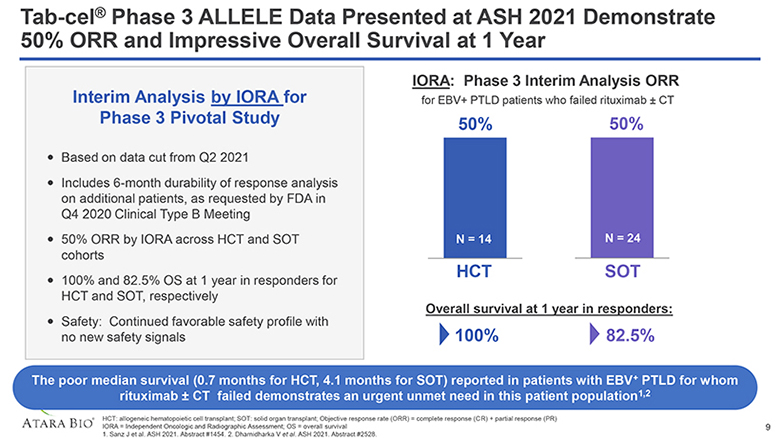
Tab-cel® Phase 3 ALLELE Data Presented at ASH 2021 Demonstrate 50% ORR and Impressive Overall Survival at 1 Year Interim Analysis by IORA for Phase 3 Pivotal Study Based on data cut from Q2 2021 Includes 6-month durability of response analysis on additional patients, as requested by FDA in Q4 2020 Clinical Type B Meeting 50% ORR by IORA across HCT and SOT cohorts 100% and 82.5% OS at 1 year in responders for HCT and SOT, respectively Safety: Continued favorable safety profile with no new safety signals IORA: Phase 3 Interim Analysis ORR for EBV+ PTLD patients who failed rituximab ± CT 50% N = 14 HCT 50% N = 24 SOT Overall survival at 1 year in responders: 100% 82.5% The poor median survival (0.7 months for HCT, 4.1 months for SOT) reported in patients with EBV+ PTLD for whom rituximab ± CT failed demonstrates an urgent unmet need in this patient population1,2 ATARA BIO® HCT: allogeneic hematopoietic cell transplant; SOT: solid organ transplant; Objective response rate (ORR) = complete response (CR) + partial response (PR) IORA = Independent Oncologic and Radiographic Assessment; OS = overall survival 1. Sanz J et al. ASH 2021. Abstract #1454. 2. Dharnidharka V et al. ASH 2021. Abstract #2528. 9
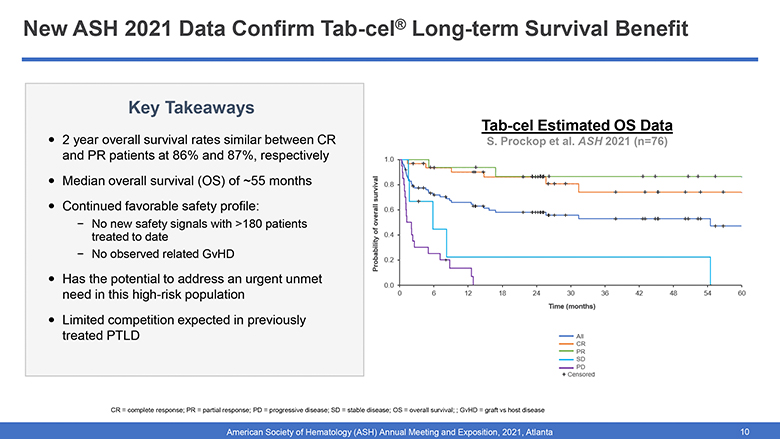
New ASH 2021 Data Confirm Tab-cel® Long-term Survival Benefit Key Takeaways 2 year overall survival rates similar between CR and PR patients at 86% and 87%, respectively Median overall survival (OS) of ~55 months in both CR and PR groups Continued favorable safety profile: No new safety signals with >180 patients treated to date No observed related GvHD Has the potential to address an urgent unmet need in this high-risk population Limited competition expected in previously treated PTLD Tab-cel OS Data Presented at ASH 2021 Probability of overall survival 1.0 0.8 0.6 0.4 0.2 0.0 0 6 12 18 24 30 36 42 48 54 60 Time (months) All CR PR SD PD + Censored CR = complete response; PR = partial response; PD = progressive disease; SD = stable disease; OS = overall survival; ; GvHD = graft vs host disease American Society of Hematology (ASH) Annual Meeting and Exposition, 2021, Atlanta 10
Atara Strategic Priorities to Create Value: ATA188 Tab-cel® (tabelecleucel) ATA188 EBV T-cell immunotherapy for progressive multiple sclerosis (MS) Fast Track designations in non-active PPMS and non-active SPMS CAR T ATARA BIO® 11

We Have Received Fast Track Designation from FDA and are Rapidly Advancing our Phase 2 EMBOLD Study for ATA188 FDA Registrational Feedback Received Fast track designation received: FDA granted fast track designation in both non-active SPMS and non-active PPMS patient populations Patient Population: FDA considers non-active SPMS and non-active PPMS as two distinct populations with high unmet medical need Next Steps: Further dialogue with FDA following interim analysis Planned Development Strategy for RCT and Phase 3 Pivotal Study Ongoing Robust Phase 2 progressing well: Key stepping stone before Phase 3 pivotal studies, with enrollment of patient 80 expected soon after the IA Phase 3 pivotal studies: to be conducted in both non-active SPMS and PPMS populations One Phase 3 study will focus on non-active SPMS, for which no approved therapies currently exist in U.S. or E.U. A separate study will focus on non-active PPMS, which has very few treatment options in most countries and approved therapeutic options are of limited efficacy Stirred-tank bioreactor: We continue to invest in this manufacturing technology to enable clinical supply and biologic-like COGM at commercial scale ATARA BIO® RCT = Randomized Controlled Trial COGM = Cost of Goods Manufactured SPMS = Secondary Progressive Multiple Sclerosis PPMS = Primary Progressive Multiple Sclerosis 12

We are Advancing Towards the Planned Interim Analysis for the ATA188 EMBOLD Study in Q2 2022 Planned Interim Analysis on Track for Q2 2022 We plan to conduct a formal interim analysis in Q2 2022, including efficacy and safety, to optimize the likelihood of success in Phase 2, and confirm current development strategy We plan to communicate our decision on next steps for the program, including rationale, while still maintaining the integrity of the study Key Aspects of Planned Interim Analysis Key data point at time of interim analysis is EDSS improvement at 6 months, for applicable patients As patients continue to enroll, there will be a range of treatment durations at the time of the IA Based on Phase 1 data: EDSS improvement at 6 months is >85% predictive of achieving EDSS improvement at months - Note: 33% EDSS improvement at 6 months in high dose cohorts in Phase 1 Results will determine sample size necessary to achieve target conditional power at end of the study and will inform Phase 3 design and planning Continued strong interest from large pharma companies on potential partnering opportunities ATARA BIO® 13
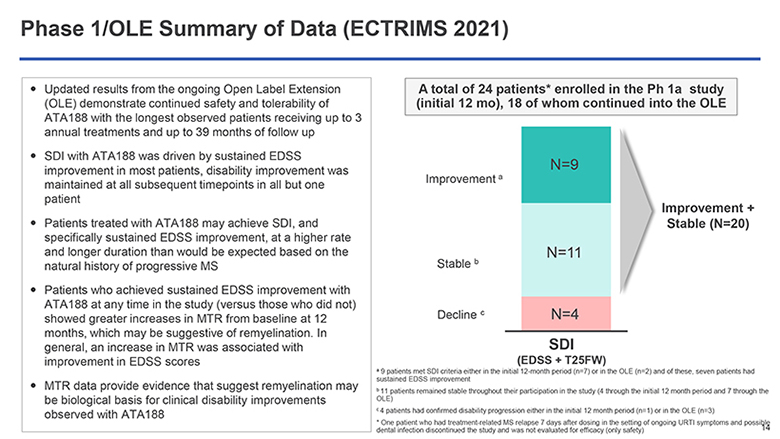
Phase 1/OLE Summary of Data (ECTRIMS 2021) Updated results from the ongoing Open Label Extension (OLE) demonstrate continued safety and tolerability of ATA188 with the longest observed patients receiving up to 3 annual treatments and up to 39 months of follow up SDI with ATA188 was driven by sustained EDSS improvement in most patients, disability improvement was maintained at all subsequent timepoints in all but one patient Patients treated with ATA188 may achieve SDI, and specifically sustained EDSS improvement, at a higher rate and longer duration than would be expected based on the natural history of progressive MS Patients who achieved sustained EDSS improvement with ATA188 at any time in the study (versus those who did not) showed greater increases in MTR from baseline at 12 months, which may be suggestive of remyelination. In general, an increase in MTR was associated with improvement in EDSS scores MTR data provide evidence that suggest remyelination may be biological basis for clinical disability improvements observed with ATA188 A total of 24 patients* enrolled in the Ph 1a study (initial 12 mo), 18 of whom continued into the OLE Improvement a Stable b Decline c N=9 N=11 N=4 Improvement + Stable (N=20) SDI (EDSS + T25FW) a 9 patients met SDI criteria either in the initial 12-month period (n=7) or in the OLE (n=2) and of these, seven patients had sustained EDSS improvement b 11 patients remained stable throughout their participation in the study (4 through the initial 12 month period and 7 through the OLE) c 4 patients had confirmed disability progression either in the initial 12 month period (n=1) or in the OLE (n=3) * One patient who had treatment-related MS relapse 7 days after dosing in the setting of ongoing URTI symptoms and possible dental infection discontinued the study and was not evaluated for efficacy (only safety) 14
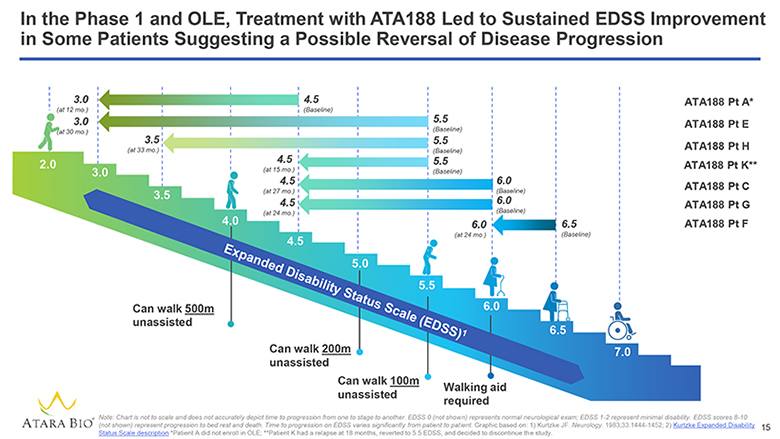
In the Phase 1 and OLE, Treatment with ATA188 Led to Sustained EDSS Improvement in Some Patients Suggesting a Possible Reversal of Disease Progression 3.0 (at 12 mo.) 3.0 (at 30 mo.) 3.5 (at 33 mo.) 4.5 (at 15 mo.) 4.5 (at 27 mo.) 4.5 (at 24 mo.) 4.5 (Baseline) 5.5 (Baseline) 5.5 (Baseline) 5.5 (Baseline) 6.0 (at 24 mo.) 6.0 (Baseline) 6.0 (Baseline) 6.5 (Baseline) 2.0 3.0 3.5 4.0 4.5 5.0 5.5 6.0 6.5 7.0 Expanded Disability Status Scale (EDSS)1 ATA188 Pt A* ATA188 Pt E ATA188 Pt H ATA188 Pt K** ATA188 Pt C ATA188 Pt G ATA188 Pt F Can walk 500m unassisted Can walk 200m unassisted can walk 100m unassisted Walking aid required ATARA BIO® Note: Chart is not to scale and does not accurately depict time to progression from one to stage to another. EDSS 0 (not shown) represents normal neurological exam; EDSS 1-2 represent minimal disability. EDSS scores 8-10 (not shown) represent progression to bed rest and death. Time to progression on EDSS varies significantly from patient to patient. Graphic based on: 1) Kurtzke JF. Neurology. 1983;33:1444-1452; 2) Kurtzke Expanded Disability Status Scale description *Patient A did not enroll in OLE; **Patient K had a relapse at 18 months, reverted to 5.5 EDSS, and decided to discontinue the study. 15
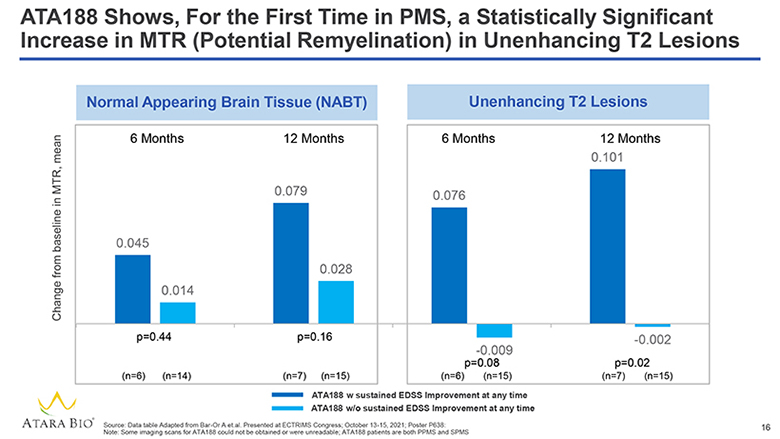
ATA188 Shows, For the First Time in PMS, a Statistically Significant Increase in MTR (Potential Remyelination) in Unenhancing T2 Lesions Change from baseline in MTR, mean Normal Appearing Brain Tissue (NABT) 6 Months 12 Months 0.045 0.014 0.079 0.028 p=0.44 p=0.16 (n=6) (n=14) (n=7) (n=15) Unenhancing T2 Lesions 6 Months 12 Months 0.076 0.101 -0.009 p=0.08 (n=6) (n=15) -0.002 p=0.02 (n=7) (n=15) ATA188 w sustained EDSS Improvement at any time ATA188 w/o sustained EDSS Improvement at any time ATARA BIO® Source: Data table Adapted from Bar-Or A et.al. Presented at ECTRIMS Congress; October 13-15, 2021; Poster P638: Note: Some imaging scans for ATA188 could not be obtained or were unreadable; ATA188 patients are both PPMS and SPMS 16
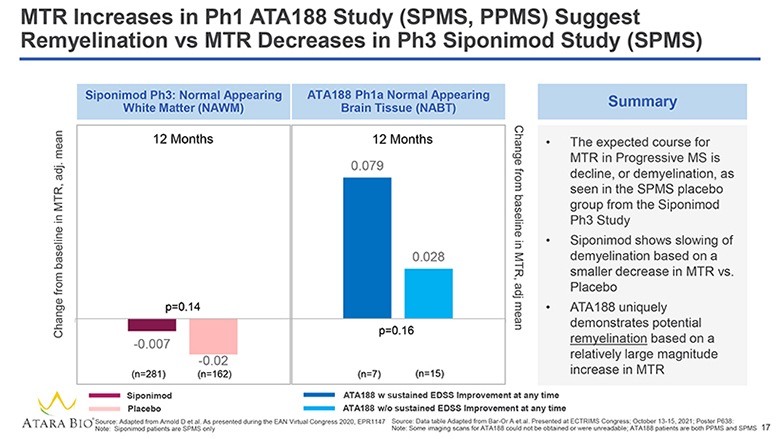
MTR Increases in Ph1 ATA188 Study (SPMS, PPMS) Suggest Remyelination vs MTR Decreases in Ph3 Siponimod Study (SPMS) Change from baseline in MTR, adj. mean Siponimod Ph3: Normal Appearing White Matter (NAWM) 12 Months p=0.14 -0.007 (n=281) -0.02 (n=162) ATA188 Ph1a Normal Appearing Brain Tissue (NABT) 12 Months 0.079 0.028 p=0.16 (n=7) (n=15) Change from baseline in MTR, adj mean Summary The expected course for MTR in Progressive MS is decline, or demyelination, as seen in the SPMS placebo group from the Siponimod Ph3 Study Siponimod shows slowing of demyelination based on a smaller decrease in MTR vs. Placebo ATA188 uniquely demonstrates potential remyelination based on a relatively large magnitude increase in MTR Siponimod Placebo ATA188 w sustained EDSS Improvement at any time ATA188 w/o sustained EDSS Improvement at any time ATARA BIO® Source: Adapted from Arnold D et al. As presented during the EAN Virtual Congress 2020, EPR1147 Note: Siponimod patients are SPMS only Source: Data table Adapted from Bar-Or A et.al. Presented at ECTRIMS Congress; October 13-15, 2021; Poster P638: Note: Some imaging scans for ATA188 could not be obtained or were unreadable; ATA188 patients are both PPMS and SPMS 17
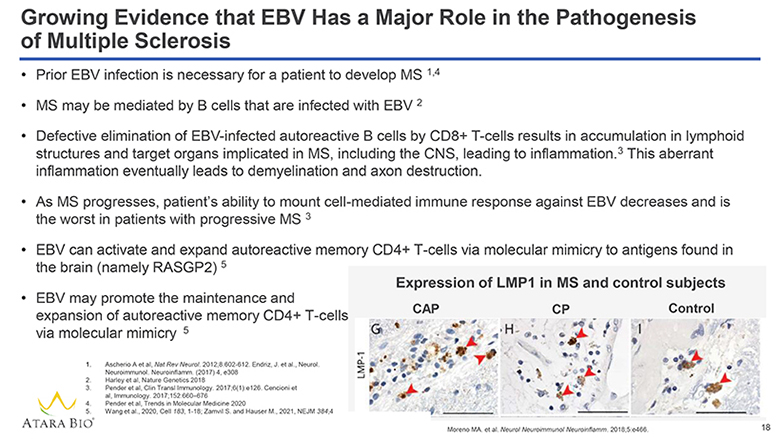
Growing Evidence that EBV Has a Major Role in the Pathogenesis of Multiple Sclerosis Prior EBV infection is necessary for a patient to develop MS 1,4 MS may be mediated by B cells that are infected with EBV 2 Defective elimination of EBV-infected autoreactive B cells by CD8+ T-cells results in accumulation in lymphoid structures and target organs implicated in MS, including the CNS, leading to inflammation. 3 This aberrant inflammation eventually leads to demyelination and axon destruction. As MS progresses, patient’s ability to mount cell-mediated immune response against EBV decreases and is the worst in patients with progressive MS 3 EBV can activate and expand autoreactive memory CD4+ T-cells via molecular mimicry to antigens found in the brain (namely RASGP2) 5 EBV may promote the maintenance and expansion of autoreactive memory CD4+ T-cells via molecular mimicry 5 1. Ascherio A et al, Nat Rev Neurol. 2012;8:602-612. Endriz, J. et al., Neurol. Neuroimmunol. Neuroinflamm. (2017) 4, e308 2. Harley et al, Nature Genetics 2018 3. Pender et al, Clin Transl Immunology. 2017;6(1):e126. Cencioni et al, Immunology. 2017;152:660–676 4. Pender et al, Trends in Molecular Medicine 2020 5. Wang et al., 2020, Cell 183, 1-18; Zamvil S. and Hauser M., 2021, NEJM 384;4 ATARA BIO® Expression of LMP1 in MS and control subjects CAP CP Control LMP-1 G H I Moreno MA. et al. Neurol Neuroimmunol Neuroinflamm. 2018;5:e466. 18
Atara Strategic Priorities to Create Value: CAR T Tab-cel® (tabelecleucel) ATA183 CAR T ATA2271/ATA3271 (Solid Tumors) ATA3219 (B-cell malignancies) Other CAR T ATARA BIO® 19
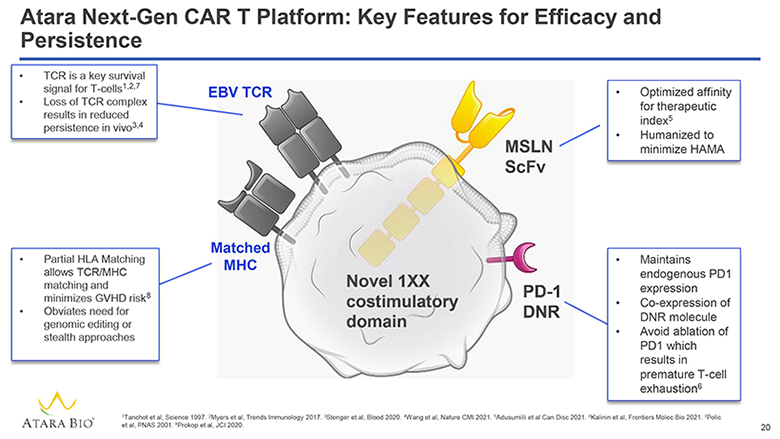
Atara Next-Gen CAR T Platform: Key Features for Efficacy and Persistence TCR is a key survival signal for T-cells1,2,7 Loss of TCR complex results in reduced persistence in vivo3,4 EBV TCR Partial HLA Matching allows TCR/MHC matching and minimizes GVHD risk8 Obviates need for genomic editing or stealth approaches Matched MHC Novel 1XX costimulatory domain MSLN ScFv Optimized affinity for therapeutic index5 Humanized to minimize HAMA PD-1 DNR Maintains endogenous PD1 expression Co-expression of DNR molecule Avoid ablation of PD1 which results in premature T-cell exhaustion6 ATARA BIO® 1Tanchot et al, Science 1997. 2Myers et al, Trends Immunology 2017. 3Stenger et al, Blood 2020. 4Wang et al, Nature CMI 2021. 5Adusumilli et al Can Disc 2021. 6Kalinin et al, Frontiers Molec Bio 2021. 7Polic et al, PNAS 2001. 8Prokop et al, JCI 2020. 20
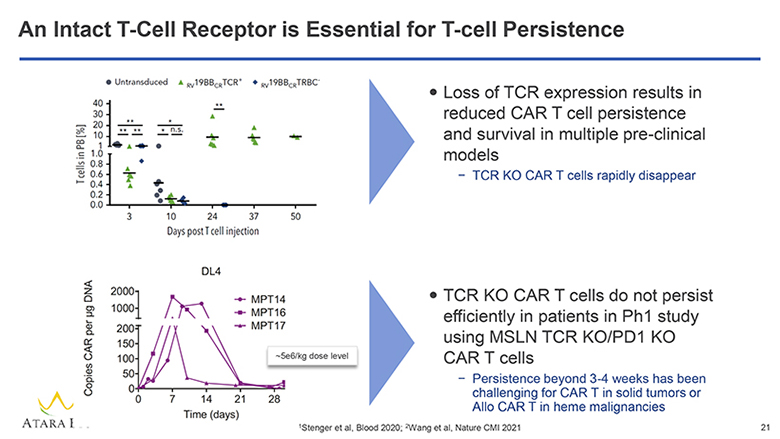
An Intact T-Cell Receptor is Essential for T-cell Persistence Untransduced RV19BBCRTCR+ RV19BBCRTRBC- T cells in PB[%] 40 30 20 10 1 1.0 0.8 0.6 0.4 0.2 0.0 3 10 24 37 50 Days post T cell injection Loss of TCR expression results in reduced CAR T cell persistence and survival in multiple pre-clinical models – TCR KO CAR T cells rapidly disappear DL4 MPT14 MPT16 MPT17 Copies CAR per ug DNA 2000 1000 200 150 100 50 0 0 7 14 21 28 Time (days) 5e6/kg dose level TCR KO CAR T cells do not persist efficiently in patients in Ph1 study using MSLN TCR KO/PD1 KO CAR T cells – Persistence beyond 3-4 weeks has been challenging for CAR T in solid tumors or Allo CAR T in heme malignancies ATARA BIO® 1Stenger et al, Blood 2020; 2Wang et al, Nature CMI 2021 21
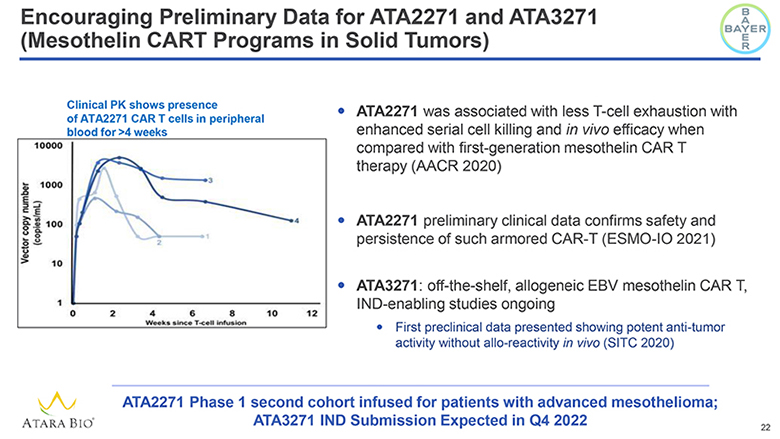
Encouraging Preliminary Data for ATA2271 and ATA3271 (Mesothelin CART Programs in Solid Tumors) BAYER Clinical PK shows presence of ATA2271 CAR T cells in peripheral blood for >4 weeks Vector copy number (copies/mL) 10000 1000 100 10 1 0 2 4 6 8 10 12 Weeks since T-cell infusion ATA2271 was associated with less T-cell exhaustion with enhanced serial cell killing and in vivo efficacy when compared with first-generation mesothelin CAR T therapy (AACR 2020) ATA2271 preliminary clinical data confirms safety and persistence of such armored CAR-T (ESMO-IO 2021) ATA3271: off-the-shelf, allogeneic EBV mesothelin CAR T, IND-enabling studies ongoing First preclinical data presented showing potent anti-tumor activity without allo-reactivity in vivo (SITC 2020) ATARA BIO® ATA2271 Phase 1 second cohort infused for patients with advanced mesothelioma; ATA3271 IND Submission Expected in Q4 2022 22
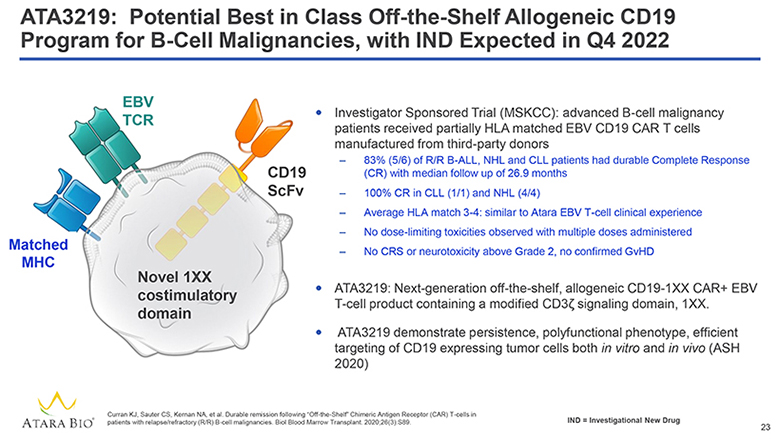
ATA3219: Potential Best in Class Off-the-Shelf Allogeneic CD19 Program for B-Cell Malignancies, with IND Expected in Q4 2022 EBV TCR Matched MHC CD19 ScFv Novel 1XX costimulatory domain Investigator Sponsored Trial (MSKCC): advanced B-cell malignancy patients received partially HLA matched EBV CD19 CAR T cells manufactured from third-party donors 83% (5/6) of R/R B-ALL, NHL and CLL patients had durable Complete Response (CR) with median follow up of 26.9 months 100% CR in CLL (1/1) and NHL (4/4) Average HLA match 3-4: similar to Atara EBV T-cell clinical experience No dose-limiting toxicities observed with multiple doses administered No CRS or neurotoxicity above Grade 2, no confirmed GvHD ATA3219: Next-generation off-the-shelf, allogeneic CD19-1XX CAR+ EBV T-cell product containing a modified CD3z signaling domain, 1XX. ATA3219 demonstrate persistence, polyfunctional phenotype, efficient targeting of CD19 expressing tumor cells both in vitro and in vivo (ASH 2020) ATARA BIO® Curran KJ, Sauter CS, Kernan NA, et al. Durable remission following “Off-the-Shelf” Chimeric Antigen Receptor (CAR) T-cells in patients with relapse/refractory (R/R) B-cell malignancies. Biol Blood Marrow Transplant. 2020;26(3):S89. IND = Investigational New Drug 23
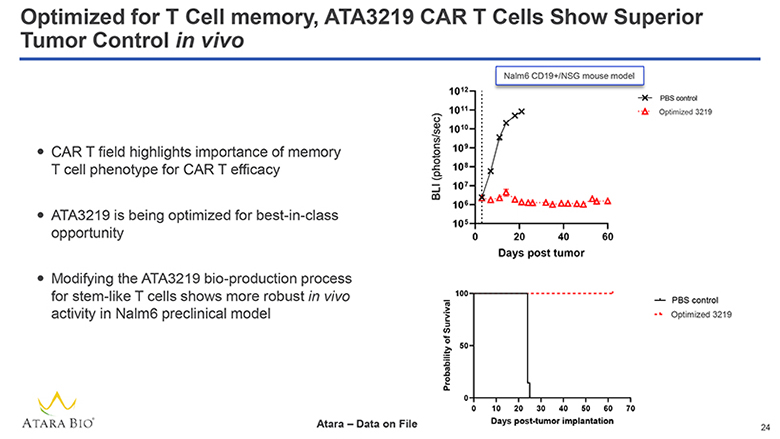
Optimized for T Cell memory, ATA3219 CAR T Cells Show Superior Tumor Control in vivo CAR T field highlights importance of memory T cell phenotype for CAR T efficacy ATA3219 is being optimized for best-in-class opportunity Modifying the ATA3219 bio-production process for stem-like T cells shows more robust in vivo activity in Nalm6 preclinical model Nalm CD19+/NSG mouse model BLI (photons/sec) 1012 1011 1010 109 108 107 106 105 0 20 40 60 PBS control Optimized 3219 Days post tumor Probability of Survival 100 50 0 0 10 20 30 40 50 60 70 PBS control Optimized 3219 Days post-tumor implantation ATARA BIO® Atara – Data on File 24
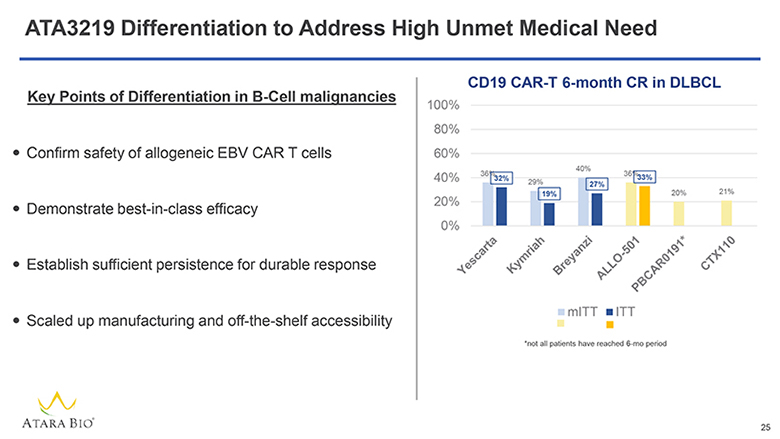
ATA3219 Differentiation to Address High Unmet Medical Need Key Points of Differentiation in B-Cell malignancies Confirm safety of allogeneic EBV CAR T cells Demonstrate best-in-class efficacy Establish sufficient persistence for durable response Scaled up manufacturing and off-the-shelf accessibility CD19 CAR-T 6-month CR in DLBCL 100% 80% 60% 40% 20% 0% Yescarta Kymriah Breyanzi ALLO-501 PBCAR0191* CTX110 mlTT lTT *not all patients have reached 6-mo period ATARA BIO® 25

We Are a Leading Allogeneic T-Cell Immunotherapy Company Differentiated Allogeneic Cell Therapy Platform Scalable EBV T-cell platform and technologies to develop multiple allogeneic cell therapies Tab-cel®: First-In-Kind, Late-Stage, Oncology Program MAA submitted in November 2021 with accelerated assessment and BLA submission expected in Q2 2022 ATA188: Potentially Transformative MS Treatment in Randomized Controlled Trial Fast-track designation granted; Placebo-controlled interim data expected in Q2 2022 to enable pivotal studies and partnering opportunities, with potential to unlock multi-billion-dollar opportunity Next-Gen Allogeneic CAR T Portfolio, Validated by Bayer Collaboration on Mesothelin-Targeted CAR T Potential best-in-class programs designed to address current limitations of autologous and allogeneic CAR T Proven Technical Capabilities Advanced process science and manufacturing capabilities within state-of-the-art facilities ATARA BIO® EBV = Epstein-Barr Virus; BLA = Biologics License Application; BTD = Break Through Designation 26